Production of Silica Gels: Alkoxide Method
Production of Silica Gels
Silica gels are produced through the sol-gel process, in which nanoparticles suspended in a liquid solution (i.e., a sol) are invoked to interconnect and form a continuous, porous, nanostructured network of particles across the volume of the liquid medium (i.e., a gel). See How Aerogel is Made for more information.
The Most Common Technique: Silicon Alkoxide Gelation
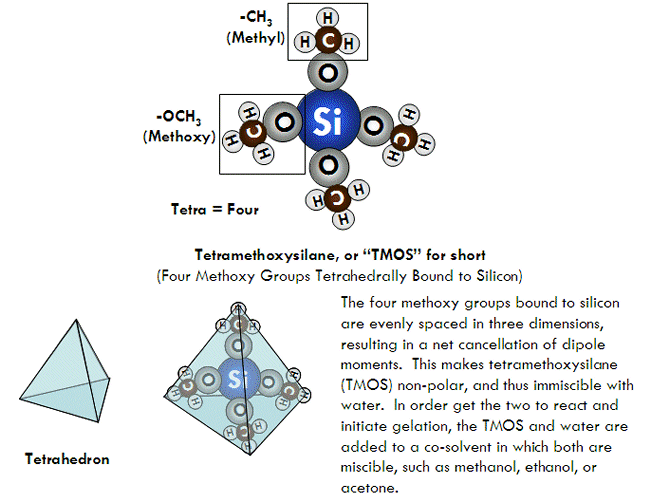
The most common technique used for producing silica gels today involves the reaction of a silicon alkoxide with water in a solvent such as ethanol or acetone, usually in the presence of basic, acidic, and/or fluoride-containing catalyst. In this technique, a silicon alkoxide (usually either tetramethoxysilane or tetraethoxysilane) serves as the source for the silica, water acts as a reactant to help join the alkoxide molecules together, and a catalyst (such as ammonium hydroxide or ammonium fluoride) helps the underlying chemical reactions go fast enough to be practically useful. Because silicon alkoxides are usually non-polar liquids, however, they are not miscible with water. As a result, a solvent such as ethanol or acetone, which is miscible with both silicon alkoxides and water, is added in order to get everything into the same phase so the necessary chemical reactions can occur.
In general, this type of chemistry can be done at ambient temperature and pressure simply by mixing the correct proportions of chemicals together—liquid goes in, gel comes out! Of course, different processes exist which are more complex this, each having their own advantages. Below is a walk-through of what happens at the molecular level when a silicon alkoxide reacts with water to form a silica gel.
A Note About Silicon Alkoxides
As mentioned, the most common type of silicon alkoxides used for making silica gels are tetramethoxysilane, abbreviated “TMOS”, and tetraethoxysilane, abbreviated “TEOS”. Both are tetrahedral, non-polar molecules. TMOS is also correctly called tetramethyl orthosilicate, where “orthosilicate” means SiO44-. Similarly, TEOS can also correctly be called tetraethyl orthosilicate. This is important to know, since some chemical companies use one name over the other. Other alkoxides, such as methyltrimethoxysilane (MTMS) are sometimes used for various reasons as well, which will be discussed later.
Formation of a Sol
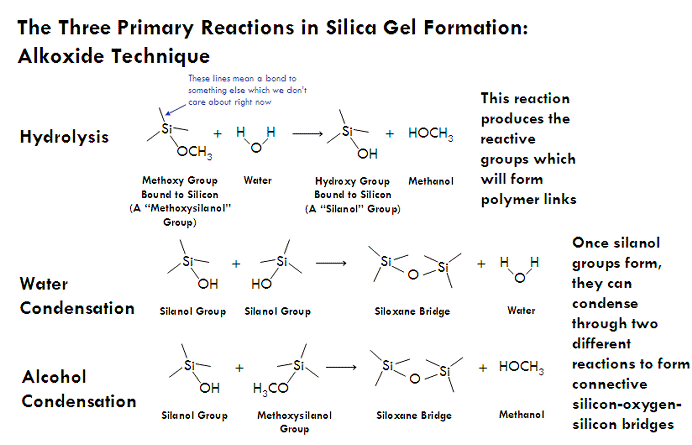
Three reactions result when an alkoxide reacts with water, together resulting in the formation of silica nanoparticles (which are what will later interconnect to form a gel). The first of these reactions is hydrolysis, in which a silicon alkoxide reacts with water to form silanol (Si-OH) groups. These silanol groups can then react either with each other or an alkoxide group (Si-OR) to form a siloxane bridge (Si-O-Si), resulting in the joining of two molecules into one larger molecule. Each silicon atom can form up to four siloxane bridges (remember, silicon is tetravalent, meaning that it is most stable forming four chemical bonds), allowing for many small molecules to connect together into giant molecules which contain thousands of silicon-oxygen bridges. These large assemblies of silicon and oxygen bridges are called silica nanoparticles, and have diameters approximately a few nanometers across.
In general, the composition of a these nanoparticles can be expressed by the empirical formula SiO2, but more accurately by the polymer formula (SiO4)n (in that each silicon atom is attached by a single bond to four oxygens which bridge to other silicon atoms). However, during the formation of a silica nanoparticle, not every silicon atom ends up forming four siloxane bridges and instead hangs on to one or more of its hydroxyl (-OH) or alkoxy (-OR) groups (which would have been used to form a siloxane bond). These are called “terminal groups”, and cover the surface of the nanoparticles.
Formation of a Gel from a Sol
At some point, the nanoparticles reach a critical size where they stop growing in size and instead agglomerate with other nanoparticles. This is dependent on a number of factors, such as the pH and concentration of other species in the solution. The terminal hydroxyl and alkoxy groups on the surface of the nanoparticles allow the nanoparticles to interconnect with each other, kind of like atomic Velcro balls.
When enough of the nanoparticles join together that a continuous network spans the liquid solution, a gel has formed. Often times gels are given special names depending on the liquid solution contained within their pores. Since an alcohol of some sort (such as methanol or ethanol) is frequently used for making silica gels, the term “silica alcogel” is often used. A “hydrogel” on the other hand would contain water in its pores.
Depending on the chemistry of the reaction solution, gelation may just happen immediately following sol formation, or may require additional catalyst, water, temperature, or time to occur. It is also possible that addition of these things will cause the nanoparticles to precipitate forming a white slush instead of a gel. As a result, the chemistry of the system can be quite sensitive.
Throughout the aerogel and, in particular, on the surface of the struts which make up the aerogel’s sponge-like framework, are functional groups where a silicon atom does not share a siloxane bridge with another silicon atom (that is, one of its four bonds does not participate in network bonding). The vast majority of these functional groups are hydroxyl (-OH) groups left over from hydrolysis of the alkoxide, and less so, alkoxy groups which were never hydrolyzed, or reformed after hydrolysis (-OR, such as methoxy).
Drawbacks of the Alkoxide Method
Although the chemistry of silicon alkoxide gelation is very straightforward and elegant from a chemical perspective, alkoxides have their drawbacks. First, alkoxides of silicon as well as alkoxides of metals are hazardous since inhaling their vapors will cause hydrolysis and thus nanoparticle formation to occur in wet tissues such as lungs and nasal membranes. Second, alkoxides tend to be very expensive since they require several chemical steps to prepare. In the case of silicon alkoxides, tetraethoxysilane is often preferred over tetramethoxysilane since it is about four times less expensive (USD$30 per liter vs. USD$120 per liter) and, although less reactive, is also less hazardous (but should still be used in a vent hood!)
But there’s more than one way to make a silica gel though!